 International Journal of Rheumatic Diseases, 2020 May;23(5):613-61.
International Journal of Rheumatic Diseases, 2020 May;23(5):613-61.
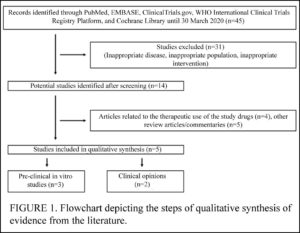
This is a review of all the (few) studies on the use of HCQ and the similar CQ for COVID-19 published by March, 2020. They searched a number of databases but only found 45 studies.
Actually, the authors only considered 5 of the studies they found — 3 were in-vitro pre-clinical studies (e.g., not on people) and 2 were opinions. The in-vitro studies showed the drug as having a prophylactic effect against COVID-19 (also known as SARS-CoV-2), and the two opinions recommended the use of HCQ and CQ for prevention of COVID-19.
Shah et al acknowledge that HCQ has a better safety profile than CQ, and is even considered safe for use in pregnancy. However, they conclude that the drug should NOT be used against COVID-19, citing unspecified “safety concerns,” “questionable efficacy,” and the “danger” of depriving current users of these drugs. They worry about creating a “false sense of protection among the common masses” if HCQ were to be used for treatment.
NOTE: Although they worry that if the drug were to be widely used, it might create a shortage, they do not consider the possibility of having the pharmaceutical companies simply make more of this inexpensive drug to fill the need.




















