 British Medical Journal. 369: m1844.
British Medical Journal. 369: m1844.
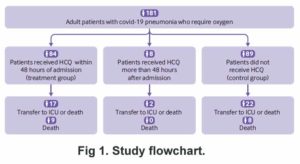
All the patients in this study were in various hospitals. They all needed oxygen and had developed pneumonia. An unknown number of the group given HCQ were not treated for the first two days after admission, and eight were not treated until an unknown number of days after that. The outcome measurement was the percent who survived until the 21st day without going to intensive care. They were about equal — 75% of the control group and 76% of the treatment group. There was a minor difference in that by day 21, 82% of the treatment group no longer needed oxygen, while only 76% of the control (untreated) group were okay without oxygen. 10% of the treatment group were removed from HCQ because they had some kind of heart-related symptom.
The dose of HCQ used was 600 mg per day. They were not given zinc and apparently were not given any sort of antibiotic, either. The authors concluded that for patients in a hospital and needing oxygen, HCQ doesn’t do much to improve their condition. They admitted they did not know if giving it earlier would be more effective.
Note: Take a look at Garcia-Cremades et al, 2020 for more on the QT with high dose HCQ. Since Garcie-Cremades et al were considering HCQ use early in the illness, it can make one wonder if the patient is more vulnerable to this side effect as he gets sicker.
Conflict of interest: In the original publication, they claimed to have no conflicts. Someone wrote to the British Medical Journal complaining, and they investigated. The authors had to include their relationship to various pharmaceutical companies, and this was published as a correction. Although none of the companies directly funded this paper, they were apparently very generous in other funding and “non-financial support.” There were SO MANY that I got tired of highlighting them. You can see the list on Page 8.




















